Peer Reviewed Journals on the Cookie Cutter Shark
Introduction
Chondrichthyes (i.due east., sharks, skates, rays, and chimeras) are amidst the most abundant and diverse vertebrate taxa (Carrier et al., 2012; Ebert et al., 2013). Among all described shark species (i.east., 537), 47% are totally or partially living in the deep-sea surroundings (i.e., beneath 200 m) (Kyne and Simpfendorfer, 2007; Cotton and Grubbs, 2015; Pollerspöck and Straube, 2019). Deep-water sharks are mainly represented in viii orders: Echinorhiniformes (100% of abyssal species), Squaliformes (86%), Hexanchiformes (86%), Pristiorhiformes (50%), Carcharhiniformes (40%), Lamniformes (20%), Squatiniformes (8%), and Orectolobiformes (4%) (Pollerspöck and Straube, 2019).
At the level of the skin epidermis, and mainly ventrally, some deep-bounding main Squaliforme sharks possess well developed lite organs (i.e., photophores) able to intrinsically emit a blue-green light (Claes and Mallefet, 2009; Renwart et al., 2014, 2015; Claes et al., 2015; Duchatelet et al., 2019a). Among Squaliformes, three families (out of the seven) contain bioluminescent species (i.e., able to emit light): the Etmopteridae, the Dalatiidae and the Somniosidae (Claes and Mallefet, 2009; Straube et al., 2015; Duchatelet et al., 2021). Shark luminescence is assumed to be mainly used for counterillumination purposes in Dalatiidae and Somniosidae, while Etmopteridae take developed an intricate blueprint of luminescent areas suggested to be used for diverse functions such every bit counterillumination, aposematism, and intraspecific communication (Claes and Mallefet, 2008; Claes et al., 2010, 2013, 2015; Duchatelet et al., 2019b, 2021; Mallefet et al., 2021). 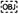 Although Somniosidae (i.e., Zameus squamulosus) luminescence remains largely enigmatic, the light emission as well as the presence and histology of photophores have been described recently (Straube et al., 2015; Duchatelet et al., 2021).
Although Somniosidae (i.e., Zameus squamulosus) luminescence remains largely enigmatic, the light emission as well as the presence and histology of photophores have been described recently (Straube et al., 2015; Duchatelet et al., 2021).
In addition to its not then common "bioluminescence part," shark skin is also known to be a pleiotropic tissue involved in a variety of functions such equally senses (e.k., Fields, 2007; Hart and Collin, 2014), protection and hydrodynamics through the placoid calibration squamation pattern (e.g., Wainwright et al., 1978; Reif, 1985; Meyer and Seegers, 2012; Oeffner and Lauder, 2012), immunity (e.thousand., Moore et al., 1993; Tsutsui et al., 2015), and color changes for camouflage or UV protection through melanophore pigment motion (e.g., Lowe and Goodman-Lowe, 1996; Visconti et al., 1999; Robbins and Fob, 2012).
The cookie-cutter shark, Isistius brasiliensis (Squaliformes, Dalatiidae) is one rare luminous shark establish in the bathy- and mesopelagic zone of the temperate and tropical waters (Jahn and Haedrich, 1987; Ebert et al., 2013; Figure 1A). With a maximum length of about 50 cm, I. brasiliensis migrates from the bounding main's deep layer to the surface at nighttime, feeding mainly on squids and small fishes (Papastamatiou et al., 2010). This shark also presents an occasional ectoparasite lifestyle, feeding on a circular-shaped clamper of mankind information technology removes from various larger pelagic organisms such every bit bony fishes (e.thousand., Jones, 1971; Muñoz-Chápuli et al., 1988; Papastamatiou et al., 2010), sharks (due east.g., Hoyos-Padilla et al., 2013), whales (due east.thou., Dwyer and Visser, 2011; Feunteun et al., 2018; Murakami et al., 2018) and seals (Le Boeuf et al., 1987). The cookie-cutter shark emits bluish light (Claes et al., 2015). On its photogenic ventral side, betwixt the gill slits and the pectoral fins, the cookie-cutter shark has a well-marked black band (also referred to as dark collar) that does not produce luminescence (Widder, 1998). Although encounters with this elusive fauna are increasing, little is known about its bioluminescence and the associated biological functions. Information lacks concerning potential similitudes (e.g., photophore morphology, low-cal emission control) with other investigated bioluminescent sharks (due east.g., Squaliolus aliae another Dalatiidae species studied past Claes et al., 2011 and Duchatelet et al., 2020a). As for all luminous sharks, the luminous compounds (i.e., luciferin and luciferase or photoprotein) underlying the light production remain unknown in this organism.
Figure one. Bioluminescence of Isistius brasiliensis. (A) Ventral side of I. brasiliensis. The dark-neckband or black band is visible on the anterior office. (B) The ventral luminous design observed on the ventral side. An absenteeism of bioluminescence is observed on the dark neckband. The dark region in the heart of the shark is artifactual and corresponds to the surface area where the specimen was stabilized for the picture. (C,D) In vivo observation of the photophores present on the ventral skin region. (E,F) Observation of the photophores using classical histology. Arrows betoken the epidermal photophores. Legend, C, connective tissue; D, dermal denticle; E, epidermis; M, muscles; I, iris-like structure; P, photocyte; Southward, pigmented sheath.
In the present study, the morphology of light-emitting photophores and their distribution along the body were investigated. In parallel, the analyses of transcriptomes of the ventral integument, the photogenic region, and the non-photogenic black ring integument region allowed usa to highlight the diversity of enzyme-coding transcripts expressed inside these functionally different tissues, with a special emphasis on the search for luciferase/photoprotein candidates.
Materials and Methods
Specimen Sampling
Adult male specimens of Isistius brasiliensis (n = three) were captured as bycatch by longliner fisherman in La Réunion Island (n = 2; registration number IS_Bmar_001 and 002) and by trawling at 1,000 thousand depth during the "Sampling the Abyss" cruise1 in June 2017 (n = 1; registration number NMV A 31829-001, Victoria Museums) operating along the declension of Commonwealth of australia and Tasmania. Living cookie-cutter shark from Australia was maintained onboard in small tank filled with common cold seawater before being euthanized by deep sedation in clove oil. The specimen collected in Australia (NMV A 31829-001, Australia) was used for in vivo observations. Integument tissues of the ventral luminous area and the black band of the same specimen were used for a pilot transcriptomic approach. In parallel, pare tissues of two individuals (IS_Bmar_001 and 002), collected in La Réunion, were used for the histological study. Due to the scarcity of this shark and the technical difficulties to isolate the epidermis from the rest of the integument layers, samples contained the upper photophore-bearing part of the skin (i.e., epidermis) as well as underlying tissues (i.e., dermis, subcutis and underlying muscles). These transcriptomes will be referred to as ventral and blackness band integument transcriptomes.
Loftier Sensitivity Macrophotography and Histology
Freshly collected individuals of I. brasiliensis were used for luminous areas detection using high sensitivity macrophotography performed in the dark. In parallel, peel patches of dissimilar areas along the torso (i.due east., rostral, mandibular, black band, pectoral, ventral, ventrolateral, dorsolateral, dorsal, dorsal fin, pelvic, caudal areas) were observed nether a calorie-free microscope and photographed. Photophore density per surface area was evaluated by counting the number of photophores per square millimeter (n = xl fields per area). Photophore diameter was too measured for each investigated area.
Patches of ventral and black ring integument (one cm2) were dissected and fixed in iv% paraformaldehyde in phosphate buffer saline (PBS) for 1 solar day at iv°C, then stored at 4°C in PBS for calorie-free microscopy analyses. Skin patches were bathed in PBS with increasing concentrations of sucrose (x% for 1 h, 20% for 1 h, and 30% overnight), before being embedded in O. C. T. chemical compound (Optimal Cutting Temperature compound, Sakura, Zoeterwude, Netherlands) and apace frozen at −80°C. Sections, ten μm in thickness, were obtained using a CM3050 Southward. Leica cryostat microtome (Frg), mounted on make clean glass slides, and left overnight to dry out. Slides were observed with a microscope (Leitz Diaplan, Germany) equipped with a ToupCam camera (UCMOS Series C-mount USB2.0 CMOS camera, ToupTek, Zhejiang, Mainland china).
RNA Extraction, cDNA Library Preparation, and Sequencing and RNA-Seq Analyses
Tissues of ventral and blackness band integument from one private were dissected and frozen in liquid nitrogen. Shark tissues were then permeabilized in RNAafter TM-Water ice (Life Technologies) overnight at −20°C, so stored at −80°C until RNA extraction procedure. Extraction of total RNA content was performed following the Trizol reagent-based protocol. RNA excerpt qualities were assessed by gel electrophoresis on a i.two% TAE agarose gel, as well as by spectrophotometry using a Nanodrop spectrometer (LabTech International). RNA quality was also checked by size-exclusion chromatography with an Agilent Technologies 2100 Bioanalyzer (Agilent RNA 6000 Nano Kit). The rarity of the species imposed this limited sampling (n = 1).
cDNA library preparation and sequencing were performed by the Beijing Genomics Found (BGI, Hong Kong) co-ordinate to the Illumina protocol (Illumina, San Diego, CA, Us). Illumina HiSeq X Ten platform was used for high-throughput sequencing to generate 150-bp paired-end reads. Raw sequences were cleaned following dissimilar filtering steps: removal of reads (i) containing the adaptor sequence just; (ii) containing over 5% of unknown nucleotides; (iii) comprising more 20% of bases with a quality value lower than 10. Read cleaning was performed using filter_fq (BGI internal software) with default settings. The remaining filtered reads were used to generate a reference de novo transcriptome assembly for I. brasiliensis, derived from the black ring and ventral integument tissues. The FastQC program (Andrews, 2010) was used to appraise the quality of the reads.
Assembly of the de novo transcriptome of I. brasiliensis was performed via short paired-end reads using the Trinity software (Grabherr et al., 2011; version release-20121005; min_contig_length 100, group_pairs_distance 250, path_reinforcement_distance 95, min_kmer_cov 2). Singled-out sequences (unigenes) were obtained, after Trinity assembly, using the TGI Clustering Tool (TGICL) (Pertea et al., 2003) post-obit a procedure described in Delroisse et al. (2015, 2016, 2018). The unigenes here grade either a group in which the similarity between overlapping sequences is greater than 94%, or singleton which represent to unmarried unigenes (Das et al., 2016). Equally the length of sequences assembled is a recognized criterion for assembly success in terms of contiguity, nosotros calculated the size distribution of both contigs and unigenes.
Based on the global unigene assembly, all the clean reads of each sample were mapped to the unigene dataset using the Bowtie2 software (v2.2.5) (Langmead and Salzberg, 2012). The gene expression levels were calculated using RSEM (v1.ii.12) (Li and Dewey, 2011). Unigene expression was expressed through the "Fragments per kilobase of transcript, per million fragments sequenced" (FPKM) values as described in Delroisse et al. (2015, 2016). It must be antiseptic that the transcriptome data have been generated in the purpose of new gene discovery, not differential expression analyses, as no biological or technical replication was performed in the framework of this study. For descriptive purposes, we identified "differentially expressed genes (DEGs)" betwixt the two transcriptomes using the "PoissonDis" method that is based on the Poisson distribution (Audic and Claverie, 1997). Unigenes for which the absolute value of log2 (Foldchange) was higher or equal to 1 and with a corrected P-value FDR inferior or equal to 0.001 were considered every bit DEGs.
Functional Gene Annotation and Transcriptome Abyss Analyses
All generated unigenes were used for homology searches against poly peptide databases (NCBI NR, Swissprot, KOG, KEGG, Interpro) using BLASTx analyses (v2.2.23, E-value < 1e–five). All-time results were selected to comment the unigenes. When the results from different databases were alien, the results from the NR database (i.e., the most complete) were preferentially selected, followed past Swissprot, KEGG and COG databases. Unigene sequences were likewise compared to nucleotide databases NT (non-redundant NCBI nucleotide database, E-value < 1e–5, BLASTn). The best aligning results of the BLASTx alignments between unigenes and protein databases like NCBI NR, Swiss-Prot, KEGG, and COG were used to identify unigenes' sequence management. When results from unlike databases are conflicting, the priority order NR, Swiss-Prot, KEGG, and COG was followed to decide on sequence direction for unigenes. The Blast2GO program (v2.5.0) was used with NR annotation to get GO annotation according to molecular function, biological process, and cellular component ontologiestwo. To farther comment the unigenes, the web Platform FunctionAnnotator3 was specifically used to annotate enzymes-coding predicted transcripts based on the PRIAM databases (E-value < 1e–x).
Transcriptome abyss was assessed using the bioinformatics tool BUSCO v4 (Benchmarking Universal Single-Copy Orthologs) to obtain the pct of unmarried-copy orthologs represented in the dataset Vertebrata_odb9. BUSCO analyses and result visualization were performed in the Milky way platform4.
Luciferase-Like Candidate Searches
To place potential luciferase/photoprotein candidates in the transcriptome of the luminous shark I. brasiliensis, reference luciferase/photoprotein sequences from various luminous organisms (e.1000., Porifera, Cnidaria, Ctenophora, Insecta, Crustacea, Echinodermata), obtained in open-access NCBI online databases were used in a "tBLASTn/reciprocal BLASTx." Reference sequences, corresponding to biochemically tested enzymes or putative enzymes described in the literature, are listed in the Supplementary Table 1. It has to be stated that some reference luciferase candidates have nevertheless to be confirmed biochemically (e.k., Arachnocampa luminosa luciferase, Suberites domuncula luciferase, Amphiura filiformis luciferase, Watasenia scintillans luciferase; Supplementary Table 1) although potent indications suggested their interest in the light emission of the considered organisms (Müller et al., 2009; Delroisse et al., 2014, 2017a,b; Gimenez et al., 2016; Watkins et al., 2018). Homologous sequences to reference luciferases/photoproteins were beginning searched using tBLASTn analyses in the newly generated integument transcriptome of I. brasiliensis. Candidate matches were then used as queries in a reciprocal BLASTx search confronting the NCBI NR online database to highlight sequences with loftier similarity with potential luciferases.
Results
High Sensitivity Photography and Histological Analyses
High sensitivity macrophotographs recorded from the cookie-cutter shark in the dark revealed a lengthened blueish glow present all over the ventral body side except at the level of the caudal fin and the area between the jaws and the pectoral fins (i.e., the then-called black band). No other specific pattern (e.1000., sexual pattern at the pelvic area, flank marking, dorsal lines) was observed. Luminescence was absent from the dorsal side (dorsal area or dorsal fins) in the tested specimen (n = 1) (Figure 1B).
In vivo observations of the different skin regions zones allowed us to identify and visualize photophores (Figures 1C,D) and to measure out their density according to the other skin areas (Supplementary Figure one). Photophores (hateful diameter = 56.1 ± v.4 μm; no statistical deviation between areas), that are scattered between the pavement-like placoid scales (Figure 1C), are present in large amount in all the studied areas except for the black band, dorsal, supra-rostral, supra-mandibular and dorsal fin areas, where but few light organs per foursquare millimeters take been observed (0.68 ± 0.47; 6.28 ± 0.14, 6.58 ± 0.74, iii.64 ± 0.33, 1.23 ± 0.41 photophore mm–2, respectively, Supplementary Figure i). Comparatively, ventral, sub-mandibular and sub-rostral areas present a photophore density of 28.75 ± 0.51, 28.xl ± 1.47 and 45.83 ± ii.17 photophore mm–2, respectively (Supplementary Figure 1). A photophore density gradient (mainly dorso-ventral, merely also antero-posterior) is clearly observed along the shark body (Supplementary Figure 1).
Transverse sections through the photogenic ventral skin of I. brasiliensis revealed the internal structure of the photophores (Figures 1E,F). Similarly to the photophores described by Seigel (1978) for the genus Squaliolus, I. brasiliensis photophores are located in the stratified squamous epidermis and are equanimous of a single photogenic cell (i.e., photocyte) embedded in a loving cup-shaped pigmented sheath, surmounted by a few cells forming an iris-similar construction (Figure 1F). Lens cells, topping the light organ, are observed in some sections.
De novo Transcriptome Sequencing, Transcriptome Quality Assessment and Annotation
A total of 88.17 and 99.60 one thousand thousand raw reads, with a length of 150 bp, were generated from the black band and ventral integument libraries, respectively. The datasets of raw reads were deposited in NCBI database: Bioproject PRJNA648842 (under SRA experiment numbers SRR12351835 and SRR12351836). After low quality reads filtering, 68.96 and fourscore.35 million high quality reads (10.34 and 12.05 Gbases in total), obtained were used to gather the blackness band and ventral integument transcriptomes with the Trinity software. Clean read quality across all bases is presented in Supplementary Figure ii. Q20 percentages (i.e., base quality more than 20) for the clean reads were higher to 78.ii% for both transcriptome datasets. Contigs were generated post-obit the overlapping information of high-quality reads. The hateful contig lengths were of 660 and 605 bp and the N50 (i.e., the length of the longest contig such that all contigs of at least that length represent at least 50% of the bases of the associates) were 1,431 and ane,154 bp for the black band and ventral integument transcriptomic data, respectively.
Contigs were further assembled into unigenes, i.eastward., unique non-redundant sequences, using paired end joining and gap filling. A total of 64,606 unigenes were obtained for the black band dataset and 43,996 for the ventral integument dataset, for a total of 68,943 different unigenes. For the unigenes, the N50 were ii,023 and i,661 bp for the black band and ventral integument transcriptomes, respectively. The unigenes length distribution is presented in the Effigy 2A. A N50 of 2,115 is reached for the global unigene dataset. The global unigene dataset includes 14,786 clusters and 54,157 singletons.
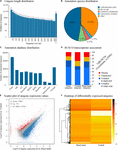
Figure 2. Isistius brasiliensis transcriptome summary. (A) Unigenes length distribution of the global transcriptome, (B) species distribution of the peak BLAST hits for all unigenes, (C) annotation summary for all investigated databases: NR, KOG, KEGG, Swissprot and Interpro. The category "intersection" refers to unigenes that are usually annotated in all databases. In contrast, the category "overall" refers to unigenes that are uniquely annotated in one of the tested databases (D) BUSCO transcriptome assessment, (Eastward) comparative unigenes expression between the blackness band and the ventral integument transcriptomes, (F) heatmap of log2 (FoldChange) of differentially expressed unigenes based on the Poisson distribution (corrected P-value FDR ≤ 0.001, | log2(FoldChange)| ≥ i) (Audic and Claverie, 1997).
On all the 68,943 I. brasiliensis pooled unigenes, 43,474 (63.06%) show meaning matches to the NCBI NR database (Supplementary Table ii, Canvass A "Unigene annotation"). Because of the lack of genome reference in I. brasiliensis and, possibly, the relatively short length of some unigenes, effectually 37% of the assembled sequences could not be matched to any known genes. Among annotated unigenes from the pooled transcriptome, 38.five% of the sequences were matched to the elephant shark Callorhinchus milii for which the genome has been sequenced (Figure 2B). On the 68,943 I. brasiliensis unigenes present in the reference transcriptome, 43,473 testify significant matches to public databases (East-value threshold > 1e–five): 36,785 to NR (53.36%), 31,628 to NT (45.88%), 32,965 to SwissProt (47.81%), 30,727 to KEGG (41.49%), 28,602 to KOG (41.49%), 29,168 to InterPro (42.31%) and 7,747 to Get (11.24%) (Figure 2C).
To evaluate the transcriptome's completeness, a BUSCO search of ii,583 vertebrate cistron groups resulted in 74.6% of consummate orthologs (including 55% of single-re-create BUSCOs and 19.6% of duplicated BUSCOs) for the global unigenes dataset. Separate analyses of both transcriptomes (i.eastward., ventral versus blackness band integuments) revealed a lower level of abyss of the ventral integument transcriptome (Figure 2D).
All the clean reads from each sample were mapped to the global unigene dataset, and the gene expression levels were calculated. On a full of 54,178 mapped unigenes, xvi,147 were only detected in the black band integument transcriptome and iii,544, only, in the ventral integument transcriptome while 34,487 were detected in both transcriptomes. For descriptive purposes, a comparative factor expression assay was performed past plotting the unigenes expression values (i.east., log10[unigenes normalized count for the black ring integument transcriptome] against log10[unigenes normalized count for the ventral integument transcriptome]), calculated for all predicted unigenes (Effigy 2E). However, as stated in the methodology, no biological or technical replication was performed as a office of the study and the present datasets have been generated in the purpose of new factor discovery just. Based on the "| logtwoFoldChange| ≥ ane" threshold and the corrected P-value FDR ≤ 0.001, 15,862 unigenes were plant to be "upregulated" in the black band integument transcriptome and 771 in the ventral integument transcriptome (Figures 2E,F).
Within the 20 most expressed unigenes of the ventral and black band integument transcriptomes (Supplementary Table ii Sheets B, C: "MostExpressed Ventral" and "MostExpressed BlackB"), predicted genes such every bit "Glyceraldehyde-3-phosphate dehydrogenase," "Fructose-bisphosphate aldolase A" are specifically represented. Both predicted transcripts coding for enzymes involved in the glycolytic pathway are possibly expressed within the muscular layer present below the skin layers. Several genes coding for muscle poly peptide actors are well represented such as "Actin alpha cardiac musculus 1-like," "Myosin regulatory light concatenation two, skeletal muscle isoform," "Tropomyosin alpha-1 chain isoform X1," "creatine kinase M-type," "Sarcoplasmic/endoplasmic reticulum calcium ATPase1" and diverse myosins. Other potential muscle markers are highly expressed in the ventral integument transcriptome (e.g., "Beta-enolase") or in the blackness band transcriptome (e.grand., "Telethonin"). Globally, it indicates a strong representation of musculus-specific genes expressed within the underlying muscle layer, perchance due to a facilitated mRNA extraction from muscle tissue compared to other tissue types from the integument.
Comparative unigene expression analyses, based on the FPKM value comparisons, indicates that within the twenty most differentially expressed unigenes in the ventral integument transcriptome (Supplementary Table 2, Sail D: "MostDiffExpressed Ventral"), many muscle specific markers tin as well exist found ("myosin-four isoform X1," "myosin regulatory light chain 2, skeletal muscle isoform," "myosin-binding protein C, fast-type isoform X1," "myosin-binding protein H-like isoform X2," "tropomodulin 4, muscle," "troponin T, cardiac muscle isoforms-like isoform X1," "myosin, heavy chain ii, skeletal muscle, adult," "CAVP-target proteins"). Other unigenes stand for to "2-acylglycerol O-acyltransferase 2-like," "immunoglobulin-similar and fibronectin type III domain-containing protein i isoform X2," "parvalbumin, thymic," "immunoglobulin-like and fibronectin type 3 domain-containing protein i isoform X1" and "PDZ and LIM domain protein five isoform X11." Conversely, other muscle-specific markers are found within the twenty almost differentially expressed unigenes in the black band transcriptome such as "tropomyosin alpha-ane chain," "tropomyosin alpha-four chain isoform X1," "myosin-7-like and troponin T, slow skeletal muscle isoform X2" (Supplementary Table 2, Sheet E: "MostDiffExpressed BlackBand"). Other unigenes, within the twenty most differentially expressed unigenes in the black band integument transcriptome, correspond to "calmodulins-like," "kelch-similar protein," "immunoglobulin superfamily fellow member 22," "homeobox poly peptide BarH-similar ii," "keratin—blazon 1 cytoskeletal 18-like isoform X1," "keratin—blazon Ii cytoskeletal 8-similar" and "profilin-2-similar isoform X3" (Supplementary Table ii, Canvass E: "MostDiffExpressed BlackBand").
Enzymatic Diverseness Evaluation Using PRIAM Notation
Based on Nail analyses performed using the PRIAM database as a reference, enzyme note was performed and allowed to place transcripts coding for 346 oxidoreductases (Form EC 1.), 1,296 transferases (Class EC ii.), 937 hydrolases (Class EC iii.), 111 lyases (EC four.), 101 isomerases (Class EC v.), 163 ligases (Course EC half dozen.), and 0 translocases (Class EC 7.) for a total of two,954 enzyme-coding transcripts for the general reference transcriptome (merged transcriptome) (Supplementary Table 3). On the 2,954 predicted enzyme-coding unigenes, xxx were uniquely establish in the photogenic ventral skin transcriptome (i.e., absent from the blackness ring transcriptome): three oxidoreductases, fourteen transferases, eleven hydrolases, 1 lyase, and 1 isomerase. The same analysis was performed on the recently published ventral integument transcriptome of the lanternshark Etmopterus spinax (Delroisse et al., 2018) and allowed to identify a like enzyme diversity: 302 oxidoreductases (Class EC 1.), ane,334 transferases (Class EC 2.), 876 hydrolases (Class EC 3.), 109 lyases (EC iv.), 95 isomerases (Class EC five.), 149 ligases (Form EC vi.), and 0 translocases (Class EC 7.) for a full of 2,865 enzyme-coding transcripts.
The Search of Transcripts Coding for Luciferase-Like Candidates
Blast analyses were used to place transcripts coding for proteins similar to leaner-blazon luciferases, insect-type luciferases (also characterizing the luminous cephalopod Watasenia and the Porifera Suberites) and to symplectin, a photoprotein isolated from the cephalopod Sthenoteuthis oulaniensis (Fujii et al., 2002). Nonetheless reciprocal Boom analyses revealed that these transcripts have more similarities to other related proteins, respectively, a «bones proline-rich protein-like», an «acyl-CoA synthetase family fellow member 2, mitochondrial» and a «biotinidase isoform X1» (Supplementary Table i). Only the "acyl-CoA synthetase family member 2, mitochondrial" appears to have a higher FPKM expression value in the ventral skin integument transcriptome. However, it is known that this metabolic enzyme is nowadays in all multicellular organisms (Inouye, 2010) and that it was specifically co-opted into a luciferase in the insect lineages, in cephalopods and possibly in sponges (Müller et al., 2009; Gimenez et al., 2016; Delroisse et al., 2017b). These analyses support the hypothesis that I. brasiliensis is using a non-described biochemical system based on an unknown luciferase/photoprotein organization.
Give-and-take
Deep-bounding main sharks exhibit various luminous patterns in terms of shape and color. Every bit previously observed (Bennett, 1840; Widder, 1998), I. brasiliensis is shown to emit light ventrally, except at the level of the highly pigmented black band expanse. The bioluminescence color range of multiple deep-sea sharks is depicted in the literature (for review in Herring, 1983; Claes and Mallefet, 2009; Renwart et al., 2014; Claes et al., 2015; Duchatelet et al., 2019a): eastward.g., Etmopterus spinax, λmax = 486 nm; East. molleri, λmax = 477 nm; E. splendidus, λmax = 476 nm; Squaliolus aliae, λmax = 457 nm; Isistius brasiliensis, λmax = 455 nm. Although the cookie cutter shark I. brasiliensis was sometimes depicted equally a greenish light emitter (Widder, 1998), our pictures of living specimens ostend a bluish luminescence consequent with the maximum wavelength of emission measured (i.e., 455 nm).
The nowadays written report highlights that photophores are almost absent (i.eastward., very few residuals photophores were observed) from the cookie cutter shark black band area equally suggested by Widder (1998). In parallel, this written report presents the starting time analysis of the photophore morphology and coverage in I. brasiliensis (and, more mostly, in Dalatiidae). As recently observed in other luminous sharks (Claes and Mallefet, 2009; Claes et al., 2015; Duchatelet et al., 2020b; Mallefet et al., 2021), I. brasiliensis photophores, visible equally blackness dots between placoid scales, are mainly distributed within the ventral epidermis with a clear dorso-ventral gradient. Counterillumination (i.e., ventral calorie-free emission that mimic the balance downwelling lite in terms of intensity, wavelength and angular distribution to avoid existence spotted past underneath swimming predators; Clarke, 1963; Johnsen et al., 2004), as an anti-predatory tool, was suggested to be the bioluminescence function for Dalatiidae species (Widder, 1998, Claes et al., 2015). Our results suggest that, conversely to the Etmopteridae luminous design (Claes and Mallefet, 2008; Claes et al., 2010, 2013, 2015; Duchatelet et al., 2019b), the cookie-cutter shark did not testify any specific pattern in the sexual areas, the flank marking, or the spine-gratis dorsal fins which back up the hypothesis of a potentially unique counterillumination part of the light product in this species. Some authors also assumed that I. brasiliensis might use its non-luminescent black band, which looks as a small fish or crustacean in a downwelling light context where the remaining body part is hidden by counterillumination, to lure larger predator (such as bigger pelagic fish, cetaceans, sharks and seals) and to steal a slice of flesh from them via its adapted jaw (Widder, 1998).
Our analyses revealed a morphological organization of the photophore very like to that observed in the genus Squaliolus (Dalatiidae, Seigel, 1978) and Zameus (Somniosidae, Duchatelet et al., 2021): a unique photocyte embedded in a pigmented sheath and surmounted by a small iris-like structure and lens cells. Therefore, an evolutive conservation of the light organ morphology is suggested in the families Dalatiidae and Somniosidae. Comparatively, the Etmopteridae shark family displays a more than complex organization with multiple photocytes per photophores (Claes and Mallefet, 2009; Renwart et al., 2014; Claes et al., 2015; Duchatelet et al., 2019a).
In parallel, this study presents a offset release of transcriptomic information for the species. Two transcriptomes were generated from integument fragments obtained from the black band region and the ventral skin region of one individual of I. brasiliensis. Several decades of inquiry focused on more accessible shallow water-sharks, and genomic surveys and genetic data are bachelor for just a few species. The complete genomes of Callorhinchus milii (Venkatesh et al., 2014), Rhincodon typus (Read et al., 2017), Chiloscyllium punctatum and Scyliorhinus torazame (Hara et al., 2018) enhanced the research on the origin of Gnathostomes and Vertebrates. The first transcriptome dataset of a deep-sea shark (Etmopterus spinax) has only recently been published (Delroisse et al., 2018) which highlight a lack of genomic/transcriptomic information for these deep-sea sharks. To our cognition, the nowadays report is the first characterization of a transcriptome of I. brasiliensis. Transcriptome annotation allowed to generate of a list of predicted transcripts expressed in the blackness ring and the ventral integument regions. These datasets could contribute to the comprehension of the gene expression of this organism and perhaps guide future research toward a better agreement of the biology of this deep-sea species.
Luciferases and photoproteins are usually not conserved amidst luminous animals. While all these enzymes perform the same biochemical task, however with various molecular substrates, non-homologous luciferases and photoproteins emerged convergently in several metazoan lineages. As an example, while insects share a common type of luciferase used for their bioluminescence (Viviani et al., 2013), three other not-homologous types of luciferase accept been described in crustaceans up to at present (i.e., Cypridina/Vargula-type luciferase, Copepoda-blazon luciferase, and Oplophorus-type luciferases) (Inouye et al., 2000; Inouye and Sasaki, 2007). In some cases, however, a homologous system appears to exist used in phylogenetically distant luminous organisms. Such a co-pick event has been strongly suggested in echinoderms (Amphiura filiformis) and urochordates (Pyrosoma atlanticum) which most likely utilise a luciferase homologous to the ane initially described in Cnidaria (Delroisse et al., 2017b; Tessler et al., 2020). In the cephalopod Watasenia, a luciferase homologous to the insect-type is used (Gimenez et al., 2016). For this reason, information technology is meaningful to search for luciferase candidates homologous to known luciferases in bioluminescent species for which the luminous system is undescribed. However, it has to be stated that a "new organization," i.e., based on a new type of enzyme not described yet, would not exist detected by this approach. This situation is observed in the well-known ostracod luciferase (Cypridina noctiluca) and the fireworm luciferase (due east.g., Odontosyllis undecimdonta) (Thompson et al., 1989; Nakajima et al., 2004; Mitani et al., 2018, 2019). Both types of luciferase indeed have no homology with known enzymes.
Luminous sharks appear equally a remaining enigma because luminous compounds (i.e., luciferin and luciferase) are still unknown (Renwart and Mallefet, 2013). In the present written report, we searched for sequences homologous to known luciferases or photoproteins in the transcriptome of I. brasiliensis. We so compared the relative expression of candidate transcripts betwixt the photogenic ventral integument and the non-photogenic blackness band integument region. Considering no specific expression profile was highlighted, our results propose that I. brasiliensis uses a new biochemical organization based on an undescribed luciferase/photoprotein system. This preliminary hypothesis, however, remains to be confirmed. Indeed, because of the presence of a very express number of photophores inside the blackness band, a strict absence of luciferase/photoprotein mRNA is unlikely in this tissue. In add-on, luciferases are classically pleiotropic proteins that may have various biochemical activities and, for that reason, may be expressed in a big multifariousness of tissues, including non-luminous ones (due east.g., Fortova et al., 2013; Delroisse et al., 2017a).
As some other way to following our hypothesis that I. brasiliensis might use an undescribed biochemical luminous system, nosotros investigated the diversity of enzymes expressed within the integument of the tested individual. Several enzyme-coding transcripts related to metabolic processes such every bit glycolysis were shown to be highly expressed and differentially expressed inside the transcriptomes. From a technical signal of view, it is particularly challenging to split peel layers from the rest of the integument without affecting the quality of the sample (i.due east., quality of extracted mRNA). For this reason, it is difficult to avoid the presence underlying tissues (i.due east., connective tissue and underlying muscles). In add-on, the RNA extractions are generally considered as efficient for musculus tissues mainly due to the high transcript expression which imply that minor deviation in terms of muscular tissue mass might atomic number 82 to strong upper or nether representation of muscle-related transcripts. This can be seen as a limitation of our dataset. However, xxx enzyme-coding unigenes were predicted to be exclusively expressed in the photogenic ventral peel transcriptome and are obviously absent from the black ring transcriptome. These 30 candidates could be the primary target of time to come investigations aiming to place the luciferase of I. brasiliensis. The generated datasets therefore constitute interesting references that should be used, in the near future, to investigate the functional characterization of the integument of this deep-sea shark.
Data Availability Statement
The datasets presented in this written report tin be found in online repositories. The names of the repository/repositories and accretion number(s) can be found below: https://world wide web.ncbi.nlm.nih.gov/, SRR12351835; https://www.ncbi.nlm.nih.gov/, SRR12351836. The refence assembled transcriptome is as well available at: https://10.0.51.84/RG.two.ii.12473.29288.
Ideals Argument
Animal procedures were conducted in compliance with Belgian national guidelines and in agreement with European directive 2010/63/UE, under the approval of the Beast Ideals Commission of the Academy of Louvain in Louvain-la-Neuve (Kingdom of belgium). Living cookie-cutter shark from Australia was maintained and euthanized co-ordinate to Museums Victoria animal intendance protocol.
Author Contributions
JD and LD performed classical histology, transcriptomic analyses, and wrote the showtime draft of the manuscript. JM collected the tissues and performed high sensitivity photography on living specimens. PF and JM supervised the work. All authors read, modified, and approved the last manuscript.
Funding
This work was supported by an FRS–FNRS Grant (T.0169.20) awarded to the Marine Biology Laboratory of the Cosmic University of Louvain (UCLouvain) and the Biology of Marine Organisms and Biomimetics Unit of the University of Mons (UMONS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Sampling the Abyss was led by Museums Victoria under Chief Scientist Tim O'Hara and supported past CSIRO Marine National Facility and NESP Marine Biodiversity Hub. Nosotros greatly thank you for the opportunity to collect Isistius brasiliensis specimens. We would likewise like to thank the reviewers for their thoughtful comments. JD, JM, and PF were, respectively, Postdoctoral Fellow, Inquiry Associate, and Research Managing director of the Fund for Scientific Inquiry of Belgium (F.R.Due south-FNRS). LD was postdoctoral researcher at the University of Louvain. This written report was a contribution from the "Centre Interuniversitaire de Biologie Marine" (CIBIM).
Supplementary Material
The Supplementary Cloth for this article tin be constitute online at: https://www.frontiersin.org/articles/ten.3389/fmars.2021.627045/total#supplementary-material
Supplementary Figure 1 | Photophore densities with the skin of I. brasiliensis.
Supplementary Figure ii | Read quality evaluation. The graph shows the quality score (Phred score) across all bases for the make clean reads obtained via Illumina 10 Ten sequencing. The box plot whiskers correspond the quantile range (between the 10 and 90% quantiles). The yellow boxes represent the inter-quartile range (betwixt the 25 and 75% quartiles). The red line is the median value. The blue line represents the mean quality.
Supplementary Tabular array one | Luciferase reference sequences and BLAST/reciprocal Blast analyses of the reference transcriptome of I. brasiliensis.
Supplementary Table two | Global transcriptome annotation (NR, NT, Go, COG, KEGG). Sheets (A–E) (A) unigene annotation, (B) 20 nigh expressed unigenes inside the ventral transcriptome, (C) 20 most expressed unigenes within the black band transcriptome, (D) 20 well-nigh differentially expressed unigenes within the ventral transcriptome, (E) 20 most differentially expressed unigenes inside the blackness band transcriptome.
Supplementary Table iii | Enzyme repertoire annotation based on the PRIAM database.
Footnotes
- ^ www.mnf.csiro.au/en/Voyages/IN2017_V03
- ^ www.geneontology.org/
- ^ www.fa.cgu.edu.tw
- ^ www.usegalaxy.eu
References
Bennett, F. D. (1840). Narrative of a Whaling Voyage Round the Globe, from the Year 1833 to 1836, Vol. two. London: Richard Bentley, 257–258.
Google Scholar
Carrier, J. F., Musick, J. A., and Heithaus, Grand. R. (eds). (2012). Biology of Sharks and their relatives. CRC Marine Biology Series. Boca Raton, FL: CRC press.
Google Scholar
Claes, J. 1000., Aksnes, D. L., and Mallefet, J. (2010). Phantom hunter of the fjords: camouflage past counterillumination in a shark (Etmopterus spinax). J. Exp. Mar. Biol. Ecol. 388, 28–32. doi: x.1016/j.jembe.2010.03.009
CrossRef Total Text | Google Scholar
Claes, J. M., and Mallefet, J. (2008). Early on development of bioluminescence suggests camouflage past counter-illumination in the velvet belly lantern shark Etmopterus spinax (Squaloidea: Etmopteridae). J. Fish Biol. 73, 1337–1350. doi: 10.1111/j.1095-8649.2008.02006.x
CrossRef Full Text | Google Scholar
Claes, J. Yard., and Mallefet, J. (2009). "Bioluminescence of sharks: first synthesis," in Bioluminescence in Focus – A Drove of Illuminating Essays, ed. V. B. Meyer-Rochow (Kerala: Research Signpost), 51–65.
Google Scholar
Claes, J. G., Dean, Thou. N., Nilsson, D.-E., Hart, N. South., and Mallefet, J. (2013). A deepwater fish with 'lightsabers' – dorsal spine-associated luminescence in a counterilluminating lanternshark. Sci. Rep. iii:1308. doi: 10.1038/srep01308
PubMed Abstruse | CrossRef Full Text | Google Scholar
Claes, J. Chiliad., Nilsson, D.-E., Straube, Due north., Collin, S. P., and Mallefet, J. (2015). Iso-luminance counterillumination drove bioluminescent shark radiation. Sci. Rep. iv, 4328. doi: x.1038/srep04328
PubMed Abstract | CrossRef Total Text | Google Scholar
Cotton wool, C. F., and Grubbs, R. D. (2015). Biological science of deep-water chondrichthyans: introduction. Deep Sea Res. ii Height. Stud. Oceanogr. 115, 1–x. doi: 10.1016/j.dsr2.2015.02.030
CrossRef Full Text | Google Scholar
Das, Southward., Shyamal, S., and Durica, D. S. (2016). Assay of annotation and differential expression methods used in RNA-Seq studies in crustacean systems. Integr. Comp. Biol. 56, 1067–1079. doi: 10.1093/icb/icw117
PubMed Abstract | CrossRef Full Text | Google Scholar
Delroisse, J., Duchatelet, L., Flammang, P., and Mallefet, J. (2018). De novo transcriptome analyses provide insights into opsin-based photoreception in the lanternshark Etmopterus spinax. PLoS One 13:e0209767. doi: ten.1371/journal.pone.0209767
PubMed Abstract | CrossRef Full Text | Google Scholar
Delroisse, J., Flammang, P., and Mallefet, J. (2014). Marine luciferases: are they really taxon-specific? A putative luciferase evolved by co-pick in an echinoderm lineage. Brilliance 29:15.
Google Scholar
Delroisse, J., Mallefet, J., and Flammang, P. (2016). De novo adult transcriptomes of two European brittle stars: spotlight on opsin-based photoreception. PLoS I 11:e0152988. doi: 10.1371/journal.pone.0152988
PubMed Abstract | CrossRef Full Text | Google Scholar
Delroisse, J., Ortega-Martinez, O., Dupont, Due south., Mallefet, J., and Flammang, P. (2015). De novo transcriptome of the European brittle star Amphiura filiformis pluteus larvae. Mar. Genomics 23, 109–121. doi: 10.1016/j.margen.2015.05.014
PubMed Abstract | CrossRef Full Text | Google Scholar
Delroisse, J., Ullrich-Lüter, E., Blaue, S., Eeckhaut, I., Flammang, P., and Mallefet, J. (2017a). Fine structure of the luminous spines and luciferase detection in the breakable star Amphiura filiformis. Zool. Anz. 269, 1–12. doi: 10.1016/j.jcz.2017.05.001
CrossRef Full Text | Google Scholar
Delroisse, J., Ullrich-Lüter, E., Blaue, Due south., Ortega-Martinez, O., Eeckhaut, I., Flammang, P., et al. (2017b). A puzzling homology: a breakable star using a putative cnidarian-type luciferase for bioluminescence. Open Biol. seven:160300. doi: 10.1098/rsob.160300
PubMed Abstract | CrossRef Total Text | Google Scholar
Duchatelet, L., Delroisse, J., Flammang, P., Mahillon, J., and Mallefet, J. (2019a). Etmopterus spinax, the velvet belly lanternshark, does not use bacterial luminescence. Acta Histochem. 121, 516–521. doi: 10.1016/j.acthis.2019.04.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Duchatelet, 50., Delroisse, J., Pinte, N., Sato, G., Ho, H.-C., and Mallefet, J. (2020a). Adrenocorticotropic hormone and circadian adenosine monophosphate are involved in the control of shark bioluminescence. Photochem. Photobiol. 96, 37–45. doi: 10.1111/php.13154
PubMed Abstract | CrossRef Full Text | Google Scholar
Duchatelet, L., Marion, R., and Mallefet, J. (2021). A third luminous shark family: confirmation of luminescence ability for Zameus squamulosus (Squaliformes; Somniosidae). Photochem. Photobiol. doi: 10.1111/php.13393 [Epub ahead of print].
CrossRef Full Text | PubMed Abstract | Google Scholar
Duchatelet, L., Pinte, N., Tomita, T., Sato, K., and Mallefet, J. (2019b). Etmopteridae bioluminescence: dorsal pattern specificity and aposematic utilise. Zool. Lett. 5:ix. doi: 10.1186/s40851-019-0126-ii
PubMed Abstruse | CrossRef Full Text | Google Scholar
Duchatelet, L., Sugihara, T., Delroisse, J., Koyanagi, 1000., Rezsohazy, R., Terakita, A., et al. (2020b). From extraocular photoreception to pigment motility regulation: a new control mechanism of the lanternshark luminescence. Sci. Rep. 10:10195. doi: 10.1038/s41598-020-67287-w
PubMed Abstruse | CrossRef Total Text | Google Scholar
Dwyer, S. L., and Visser, I. N. (2011). Cookie cutter shark (Isistius sp.) bites on cetaceans, with particular reference to killer whales (Orca) (Orcinus orca). Aquat. Mamm. 37, 111–138. doi: x.1578/AM.37.ii.2011.111
CrossRef Total Text | Google Scholar
Ebert, D. A., Fowler, South. L., and Compagno, Fifty. J. (2013). Sharks of the World: A Fully Illustrated Guide. Plymouth: Wild Nature Press.
Google Scholar
Feunteun, A., de Schrevel, C., Verhaegen, 1000., Chevallier, D., Duchemin, M., Ziani, N., et al. (2018). First evaluation of the cookie cutter sharks (Isistius sp.) predation pattern on different cetacean species in Martinique. Environ. Biol. Fish. 101, 749–759. doi: 10.1007/s10641-018-0735-i
CrossRef Total Text | Google Scholar
Fields, R. D. (2007). The shark'due south electric sense. Sci. Am. 297, 74–81.
Google Scholar
Fortova, A., Sebestova, Eastward., Stepankova, V., Koudelakova, T., Palkova, L., Damborsky, J., et al. (2013). DspA from Strongylocentrotus purpuratus: the showtime biochemically characterized haloalkane dehalogenase of non-microbial origin. Biochimie 95, 2091–2096. doi: x.1016/j.biochi.2013.07.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Fujii, T., Ahn, J. Y., Kuse, M., Mori, H., Matsuda, T., and Isobe, Thou. (2002). A novel photoprotein from oceanic squid (Symplectoteuthis oualaniensis) with sequence similarity to mammalian carbon–nitrogen hydrolase domains. Biochem. Biophys. Res. Commun. 293, 874–879. doi: ten.1016/S0006-291X(02)00296-6
CrossRef Full Text | Google Scholar
Gimenez, Yard., Metcalf, P., Paterson, N. 1000., and Sharpe, 1000. L. (2016). Mass spectrometry analysis and transcriptome sequencing reveal glowing squid crystal proteins are in the aforementioned superfamily as firefly luciferase. Sci. Rep. 6:27638. doi: ten.1038/srep27638
PubMed Abstruse | CrossRef Total Text | Google Scholar
Grabherr, K. G., Haas, B. J., Yassour, Grand., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652. doi: 10.1038/nbt.1883
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hara, Y., Yamaguchi, K., Onimaru, K., Kadota, M., Koyanagi, M., Keeley, S. D., et al. (2018). Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates. Nat. Ecol. Evol. 2, 1761–1771. doi: 10.1038/s41559-018-0673-5
PubMed Abstract | CrossRef Total Text | Google Scholar
Herring, P. J. (1983). The spectral characteristics of luminous marine organisms. Proc. R. Soc. Lond. B. 220, 183–217. doi: 10.1098/rspb.1983.0095
CrossRef Full Text | Google Scholar
Hoyos-Padilla, K., Papastamatiou, Y. P., O'Sullivan, J., and Lowe, C. K. (2013). Observation of an attack by a Cookiecutter shark (Isistius brasiliensis) on a white shark (Carcharodon carcharias). Pac. Sci. 67, 129–134. doi: 10.2984/67.1.10
CrossRef Full Text | Google Scholar
Inouye, S., and Sasaki, S. (2007). Overexpression, purification and characterization of the catalytic component of Oplophorus luciferase in the deep-sea shrimp, Oplophorus gracilirostris. Protein Exp. Purif. 56, 261–268. doi: 10.1016/j.pep.2007.08.002
PubMed Abstract | CrossRef Full Text | Google Scholar
Inouye, S., Watanabe, K., Nakamura, H., and Shimomura, O. (2000). Secretional luciferase of the luminous shrimp Oplophorus gracilirostris: cDNA cloning of a novel imidazopyrazinone luciferase. FEBS Lett. 481, 19–25. doi: 10.1016/S0014-5793(00)01963-3
CrossRef Full Text | Google Scholar
Jahn, A. Due east., and Haedrich, R. 50. (1987). Notes on the pelagic squaloid shark Isistius brasiliensis. Biol. Oceanogr. 5, 297–309. doi: ten.1080/01965581.1987.10749519
CrossRef Full Text | Google Scholar
Johnsen, S., Widder, Eastward. A., and Mobley, C. D. (2004). Propagation and perception of bioluminescence: factors affecting counterillumination as a cryptic strategy. Biol. Bull. 207, 1–16. doi: 10.2307/1543624
PubMed Abstract | CrossRef Full Text | Google Scholar
Jones, E. C. (1971). Isistius brasiliensis, a squaloid shark, the probable crusade of crater wounds on fishes and cetaceans. Fish. Bull. USA 69, 791–798.
Google Scholar
Le Boeuf, B. J., McCosker, J. Eastward., and Hewitt, J. (1987). Crater wounds on northern elephant seals: the Cookiecutter shark strikes again. Fish. Bull. 85, 387–392.
Google Scholar
Li, B., and Dewey, C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12:323. doi: 10.1186/1471-2105-12-323
PubMed Abstract | CrossRef Full Text | Google Scholar
Mallefet, J., Stevens, D. W., and Duchatelet, L. (2021). Bioluminescence of the largest luminous vertebrate, the kitefin shark, Dalatias licha: first insights and comparative aspects. Front. Mar. Sci. 8:633582. doi: 10.3389/fmars.2021.633582
CrossRef Full Text | Google Scholar
Mitani, Y., Yasuno, R., Futahashi, R., Oakley, T. H., and Ohmiya, Y. (2019). Luciferase gene of a Caribbean fireworm (Syllidae) from Puerto Rico. Sci. Rep. 9:13015.
Google Scholar
Mitani, Y., Yasuno, R., Isaka, M., Mitsuda, N., Futahashi, R., Kamagata, Y., et al. (2018). Novel gene encoding a unique luciferase from the fireworm Odontsyllis undecimdonta. Sci. Rep. 8, 12789.
Google Scholar
Moore, 1000. South., Wehrli, S., Roder, H., Rogers, Chiliad., Forrest, J. N. Jr., McCrimmon, D., et al. (1993). Squalamine: an aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. United statesA. xc, 1354–1358. doi: 10.1073/pnas.ninety.4.1354
PubMed Abstract | CrossRef Full Text | Google Scholar
Müller, W. Due east. G., Kasueske, M., Wang, Ten., Schröder, H. C., Wang, Y., Pisignano, D., et al. (2009). Luciferase a lite source for the silica-based optical waveguides (spicules) in the demosponge Suberites domuncula. Prison cell. Mol. Life Sci. 66:537. doi: 10.1007/s00018-008-8492-five
PubMed Abstract | CrossRef Full Text | Google Scholar
Muñoz-Chápuli, R., Rel Salgado, J. C., and De La Serna, J. Thousand. (1988). Biogeography of Isistius brasiliensis in the North-Eastern Atlantic, inferred from crater wounds on Swordfish (Xiphias gladius). J. Mar. Biol. Assoc. U. Chiliad. 68, 315–321. doi: ten.1017/S0025315400052218
CrossRef Full Text | Google Scholar
Murakami, C., Yoshida, H., and Yonezaki, S. (2018). Cookie-cutter shark Isistius brasiliensis eats Bryde'due south whale Balaenoptera brydei. Ichthyol. Res. 65, 398–404. doi: 10.1007/s10228-018-0619-half dozen
CrossRef Full Text | Google Scholar
Nakajima, Y., Kobayashi, K., Yamagishi, K., Enomoto, T., and Ohmiya, Y. (2004). cDNA cloning and characterization of a secreted luciferase from the luminous Japanese ostracod, Cypridina noctiluca. Biosci. Biotechnol. Biochem. 68, 565–570. doi: 10.1271/bbb.68.565
PubMed Abstract | CrossRef Full Text | Google Scholar
Papastamatiou, Y. P., Wetherbee, B. Grand., O'Sullivan, J., Goodmanlowe, 1000. D., and And Lowe, C. G. (2010). Foraging environmental of cookiecutter sharks (Isistius brasiliensis) on pelagic fishes in Hawaii, inferred from prey bite wounds. Environ. Biol. Fish. 88, 361–368. doi: 10.1007/s10641-010-9649-ii
CrossRef Full Text | Google Scholar
Pertea, Thousand., Huang, 10., Liang, F., Antonescu, V., Sultana, R., Karamycheva, South., et al. (2003). TIGR Factor indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics 19, 651–652. doi: ten.1093/bioinformatics/btg034
PubMed Abstract | CrossRef Full Text | Google Scholar
Pollerspöck, J., and Straube, Due north. (2019). Bibliography Database of Living/Fossil Sharks, Rays and Chimaeras (Chondrichthyes: Elasmobranchii, Holocephali) – Papers of the year 2018, version 01/2019. Earth Wide Web electronic publication. Available online at: www.shark-references.com (accessed December, 2020).
Google Scholar
Read, T. D., Petit, R. A., Joseph, South. J., Alam, Chiliad. T., Weil, Thousand. R., Ahmad, 1000., et al. (2017). Draft sequencing and associates of the genome of the world's largest fish, the whale shark: Rhincodon typus smith 1828. BMC Genom. 18:532. doi: 10.1186/s12864-017-3926-ix
PubMed Abstract | CrossRef Full Text | Google Scholar
Reif, W.-Eastward. (1985). Functions of scales and photophores in mesopelagic luminescent sharks. Acta Zool. 66, 111–118. doi: ten.1111/j.1463-6395.1985.tb00829.ten
CrossRef Total Text | Google Scholar
Renwart, M., and Mallefet, J. (2013). Outset written report of the chemical science of the luminous system in a deep-sea shark, Etmopterus spinax Linnaeus, 1758 (Chondrichthyes: Etmopteridae). J. Exp. Mar. Biol. Ecol. 448, 214–219. doi: 10.1016/j.jembe.2013.07.010
CrossRef Full Text | Google Scholar
Renwart, One thousand., Delroisse, J., Claes, J. M., and Mallefet, J. (2014). Ultrastructure arrangement of lantern shark (Etmopterus spinax, Linnaeus, 1758) photophores. Zoomorphology 133, 405–416. doi: ten.1007/s00435-014-0230-y
CrossRef Full Text | Google Scholar
Renwart, M., Delroisse, J., Flammang, P., Claes, J. Grand., and Mallefet, J. (2015). Cytological changes during luminescence product in lanternshark (Etmopterus spinax Linnaeus, 1758) photophores. Zoomorphology 134, 107–116. doi: 10.1007/s00435-014-0235-vi
CrossRef Total Text | Google Scholar
Robbins, R., and Fox, A. (2012). Further testify of pigmentation change in white sharks, Carcharodon carcharias. Mar. Freshw. Res. 63, 1215–1217. doi: 10.1071/MF12208
CrossRef Full Text | Google Scholar
Seigel, J. A. (1978). Revision of the Dalatiid shark genus Squaliolus: anatomy, systematics, ecology. Copeia 1978, 602–614. doi: 10.2307/1443686
CrossRef Full Text | Google Scholar
Straube, North., Li, C., Claes, J. M., Corrigan, South., and Naylor, G. J. P. (2015). Molecular phylogeny of Squaliformes and first occurrence of bioluminescence in sharks. BMC Evol. Biol. xv:162. doi: 10.1186/s12862-015-0446-half dozen
PubMed Abstract | CrossRef Full Text | Google Scholar
Tessler, M., Gaffney, J. P., Oliveira, A. Chiliad., Guarnaccia, A., Dobi, K. C., Gujarati, N. A., et al. (2020). A putative chordate luciferase from a cosmopolitan tunicate indicates convergent bioluminescence evolution across phyla. Sci. Rep. 10, one–11. doi: 10.1038/s41598-020-73446-w
PubMed Abstruse | CrossRef Total Text | Google Scholar
Thompson, E. M., Nagata, Southward., and Tsuji, F. I. (1989). Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii. Proc. Natl. Acad. Sci. 86, 6567–6571. doi: x.1073/pnas.86.17.6567
PubMed Abstract | CrossRef Full Text | Google Scholar
Tsutsui, South., Dotsuta, Y., Ono, A., Suzuki, G., Tateno, H., Hirabayashi, J., et al. (2015). A C-type lectin isolated from the skin of Japanese bullhead shark (Heterondontus japonicus) binds a remarkably broad range of sugars and induces blood coagulation. J. Biochem. 157, 345–356. doi: x.1093/jb/mvu080
PubMed Abstract | CrossRef Full Text | Google Scholar
Venkatesh, B., Lee, A. P., Ravi, V., Maurya, A. K., Lian, M. Thou., Swann, J. B., et al. (2014). Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174–179. doi: 10.1038/nature12826
PubMed Abstruse | CrossRef Total Text | Google Scholar
Visconti, M. A., Ramanzini, G. C., Camargo, C. R., and Castrucci, A. One thousand. L. (1999). Elasmobranch color alter: a short review and novel information on hormone regulation. J. Exp. Zool. 284, 485–491. doi: 10.1002/(SICI)1097-010X(19991001)284:v<485::Help-JEZ3<3.0.CO;2-five
CrossRef Full Text | Google Scholar
Viviani, V. R., Prado, R. A., Neves, D. R., Kato, D., and Barbosa, J. A. (2013). A route from darkness to light: emergence and evolution of luciferase activeness in AMP-CoA-ligases inferred from a mealworm luciferase-like enzyme. Biochemistry 52, 3963–3973. doi: 10.1021/bi400141u
PubMed Abstract | CrossRef Full Text | Google Scholar
Watkins, O. C., Sharpe, Chiliad. L., Perry, N. B., and Krause, G. L. (2018). New Zealand glowworm (Arachnocampa luminosa) bioluminescence is produced by a firefly-like luciferase but an entirely new luciferin. Sci. Rep. eight:3278. doi: 10.1038/s41598-018-21298-due west
PubMed Abstract | CrossRef Full Text | Google Scholar
Widder, Eastward. A. (1998). A predatory use of counter-illumination by the squaloid shark, Isistius brasiliensis. Environ. Biol. Fish. 53, 267–273. doi: 10.1023/A:1007498915860
CrossRef Total Text | Google Scholar
henleyquodges1936.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fmars.2021.627045/full
0 Response to "Peer Reviewed Journals on the Cookie Cutter Shark"
Post a Comment